
 |
Biological membranes comprise many different molecules that regulate shape and function. The statistical mechanics, thermodynamics, and dynamics of these complex membranes is intrinsically interesting for its fundamental physics, and can provide insight into the function and structure of cells and organelles. Aspects of interest include phase separation within membranes, coupling between composition and shape (curvature), dynamics of shape transformations, and the interplay with embedded protein dynamics. |
 |
Comment on "Elastic Membrane Deformations Govern Interleaflet Coupling of Lipid-Ordered Domains, J. J. Williamson and P. D. Olmsted, Physical Review Letters 116 (2016) 079801.
 We address a recent article: "Elastic Membrane Deformations Govern Interleaflet
Coupling of Lipid-Ordered Domains", by T. R. Galimzyanov, R. J. Molotkovsky,
M. E. Bozdaganyan, F. S. Cohen, P. Pohl, and S. A. Akimov, Phys. Rev. Lett. 115,
088101 (2015) . They calculate the tilt of lipids at the interface between
domains, and thus provide a method for calculating line tensions between phases.
They show that the interface between registered domains has a `slip layer' of
antiregistration, where each leaflet has a different phase.
We address a recent article: "Elastic Membrane Deformations Govern Interleaflet
Coupling of Lipid-Ordered Domains", by T. R. Galimzyanov, R. J. Molotkovsky,
M. E. Bozdaganyan, F. S. Cohen, P. Pohl, and S. A. Akimov, Phys. Rev. Lett. 115,
088101 (2015) . They calculate the tilt of lipids at the interface between
domains, and thus provide a method for calculating line tensions between phases.
They show that the interface between registered domains has a `slip layer' of
antiregistration, where each leaflet has a different phase.
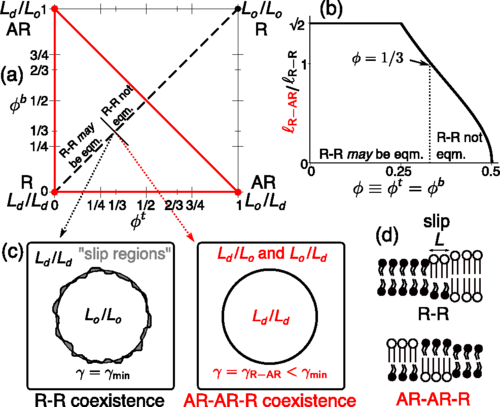
In their paper they argue that the line tension they have calculated explains domain registration between leaflets, with no need for the direct (area-dependent) inter-leaflet coupling often considered. We show that this is not quite correct, and that one needs to consider the entire line energy for given composition(involving the length of interface) in order to predict equilibrium states. When this is done properly, one tension alone does not in general explain equilibrium domain registration.
Dynamics of an asymmetric bilayer lipid membrane in a viscous solvent, R. J. Bingham, S. W. Smye, and P. D. Olmsted, Europhysics Letters 111 (2015) 18004 [Editor's Suggestion].
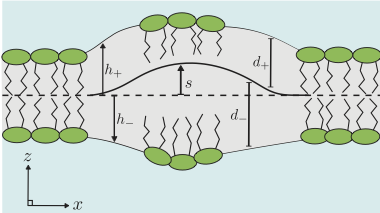 Bilayer lipid membranes (BLMs) are an
essential component of many biological systems, forming a
functional barrier between the cell and the surrounding
environment. When the membrane relaxes from a structural
perturbation, the dynamics of the relaxation depends on the
bilayer structure. We present a model of a BLM in a viscous
solvent, including an explicit description of a `thick' membrane,
where the fluctuations in the thickness of a monolayer leaflet are
coupled to changes in the lipid density within that monolayer. We
find dispersion relations describing three intuitive forms of
bilayer motion, including a mode describing motion of the
intermonolayer surface not noted previously in the literature. Two
intrinsic length scales emerge that help characterise the
dynamics; the well known Saffman-Delbruck length and another, lr,
resulting from the intermonolayer friction. The framework also
allows for asymmetry in the BLM parameters between the monolayer
leaflets, which is found to couple dynamic modes of bilayer
motion.
Bilayer lipid membranes (BLMs) are an
essential component of many biological systems, forming a
functional barrier between the cell and the surrounding
environment. When the membrane relaxes from a structural
perturbation, the dynamics of the relaxation depends on the
bilayer structure. We present a model of a BLM in a viscous
solvent, including an explicit description of a `thick' membrane,
where the fluctuations in the thickness of a monolayer leaflet are
coupled to changes in the lipid density within that monolayer. We
find dispersion relations describing three intuitive forms of
bilayer motion, including a mode describing motion of the
intermonolayer surface not noted previously in the literature. Two
intrinsic length scales emerge that help characterise the
dynamics; the well known Saffman-Delbruck length and another, lr,
resulting from the intermonolayer friction. The framework also
allows for asymmetry in the BLM parameters between the monolayer
leaflets, which is found to couple dynamic modes of bilayer
motion.
Nucleation of registered domains in the coupled leaflets of a bilayer, J. J. Williamson and P. D. Olmsted, Soft Matter 11 (2015) 8948-8959
 We study the kinetics governing the attainment of inter-leaflet domain symmetry
in a phase-separating amphiphilic bilayer. "Indirect" inter-leaflet coupling via
hydrophobic mismatch can induce an instability towards a metastable pattern of
locally asymmetric domains upon quenching from high temperature. This
necessitates a nucleation step to form the conventional symmetric domains, which
are favoured by a "direct" inter-leaflet coupling. We model the energetics for a
symmetric domain to nucleate from the metastable state, and find that an
interplay between hydrophobic mismatch and thickness stretching/compression
causes the effective hydrophobic mismatch, and thus line tension, to depend on
domain size. This leads to strong departure from classical nucleation theory. We
speculate on implications for cell membrane rafts or clusters, whose size may be
of similar magnitude to estimated critical radii for domain symmetry.
We study the kinetics governing the attainment of inter-leaflet domain symmetry
in a phase-separating amphiphilic bilayer. "Indirect" inter-leaflet coupling via
hydrophobic mismatch can induce an instability towards a metastable pattern of
locally asymmetric domains upon quenching from high temperature. This
necessitates a nucleation step to form the conventional symmetric domains, which
are favoured by a "direct" inter-leaflet coupling. We model the energetics for a
symmetric domain to nucleate from the metastable state, and find that an
interplay between hydrophobic mismatch and thickness stretching/compression
causes the effective hydrophobic mismatch, and thus line tension, to depend on
domain size. This leads to strong departure from classical nucleation theory. We
speculate on implications for cell membrane rafts or clusters, whose size may be
of similar magnitude to estimated critical radii for domain symmetry.
Kinetics of symmetry and asymmetry in a phase-separating bilayer membrane, J. J. Williamson and P. D. Olmsted, Physical Review E 92 (2015) 052721
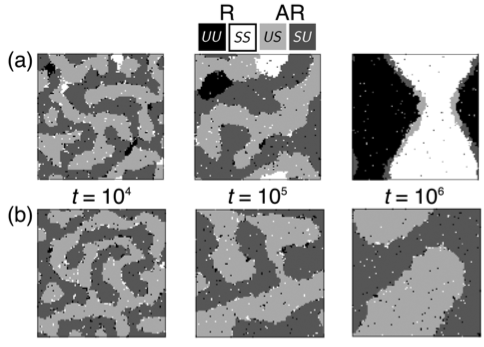
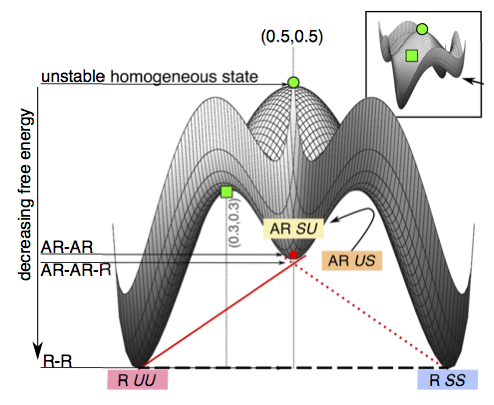 We simulate a phase-separating a bilayer in which the leaflets experience a
direct coupling favouring local compositional symmetry ("registered" bilayer
phases), and an indirect coupling due to hydrophobic mismatch that favours
strong local asymmetry ("antiregistered" bilayer phases). For wide ranges of
overall leaflet compositions, multiple competing states are possible. For
estimated physical parameters, a quenched bilayer may first evolve toward a
metastable state more asymmetric than if the leaflets were uncorrelated;\
subsequently, it must nucleate to reach its equilibrium, more symmetric, state.
These phase-transition kinetics exhibit characteristic signatures through which
fundamental and opposing inter-leaflet interactions may be probed. We emphasise
how bilayer phase diagrams with a separate axis for each leaflet can account for
overall and local symmetry/asymmetry, and capture a range of observations in the
experiment and simulation literature.
We simulate a phase-separating a bilayer in which the leaflets experience a
direct coupling favouring local compositional symmetry ("registered" bilayer
phases), and an indirect coupling due to hydrophobic mismatch that favours
strong local asymmetry ("antiregistered" bilayer phases). For wide ranges of
overall leaflet compositions, multiple competing states are possible. For
estimated physical parameters, a quenched bilayer may first evolve toward a
metastable state more asymmetric than if the leaflets were uncorrelated;\
subsequently, it must nucleate to reach its equilibrium, more symmetric, state.
These phase-transition kinetics exhibit characteristic signatures through which
fundamental and opposing inter-leaflet interactions may be probed. We emphasise
how bilayer phase diagrams with a separate axis for each leaflet can account for
overall and local symmetry/asymmetry, and capture a range of observations in the
experiment and simulation literature.
Registered and antiregistered phase separation of mixed amphiphilic bilayers, J. J. Williamson and P. D. Olmsted, Biophysical Journal 108 (2015)1963-1976 (Cover image).
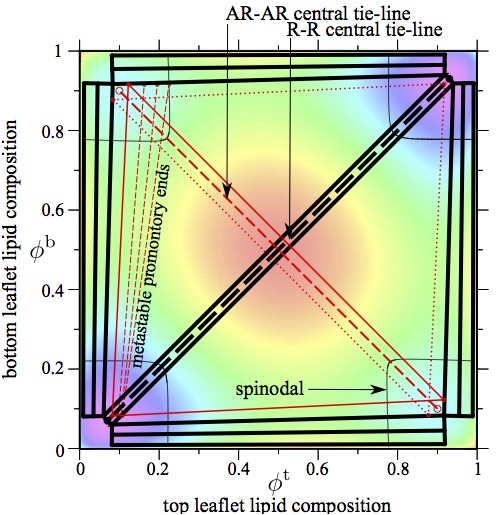 We derive a mean-field free energy for the phase behaviour of coupled
bilayer leaflets, which is implicated in cellular processes and
important to the design of artificial membranes. Our model accounts
for amphiphile-level structural features, particularly hydrophobic
mismatch, which pro- motes antiregistration (AR), in competition with
the ‘direct’ trans-midplane coupling usually stud- ied, promoting
registration (R). We show that the phase diagram of coupled leaflets
allows multiple metastable coexistences, then illustrate the kinetic
implications with a detailed study of a bilayer of equimolar overall
composition. For approximate parameters estimated to apply to phospho-
lipids, equilibrium coexistence is typically registered, but
metastable antiregistered phases can be kinetically favoured by
hydrophobic mismatch. Thus a bilayer in the spinodal region can
require nucleation to equilibrate, in a novel manifestation of
Ostwald's `rule of stages'. Our results provide a framework for
understanding disparate existing observations, elucidating a subtle
competition of couplings, and a key role for phase transition kinetics
in bilayer phase behaviour.
We derive a mean-field free energy for the phase behaviour of coupled
bilayer leaflets, which is implicated in cellular processes and
important to the design of artificial membranes. Our model accounts
for amphiphile-level structural features, particularly hydrophobic
mismatch, which pro- motes antiregistration (AR), in competition with
the ‘direct’ trans-midplane coupling usually stud- ied, promoting
registration (R). We show that the phase diagram of coupled leaflets
allows multiple metastable coexistences, then illustrate the kinetic
implications with a detailed study of a bilayer of equimolar overall
composition. For approximate parameters estimated to apply to phospho-
lipids, equilibrium coexistence is typically registered, but
metastable antiregistered phases can be kinetically favoured by
hydrophobic mismatch. Thus a bilayer in the spinodal region can
require nucleation to equilibrate, in a novel manifestation of
Ostwald's `rule of stages'. Our results provide a framework for
understanding disparate existing observations, elucidating a subtle
competition of couplings, and a key role for phase transition kinetics
in bilayer phase behaviour.
Fast cholesterol flip-flop and lack of swelling in skin lipid multilayers, Chinmay Das, Massimo Noro, and Peter D. Olmsted, Soft Matter 10 (2014) 7346.
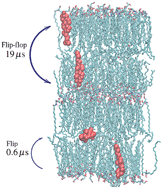 Atomistic simulations were performed on hydrated model lipid
multilayers that are representative of the lipid matrix in the outer
skin (stratum corneum). We find that cholesterol transfers easily
between adjacent leaflets belonging to the same bilayer via fast
orientational diffusion (tumbling) in the inter- leaflet disordered
region, while at the same time there is a large free energy cost
against swelling. This fast flip-flop may play an important role in
accommodating the variety of curvatures that would be required in the
three dimensional arrangement of the lipid multilayers in skin, and
for enabling mechanical or hydration induced strains without large
curvature elastic costs.
Atomistic simulations were performed on hydrated model lipid
multilayers that are representative of the lipid matrix in the outer
skin (stratum corneum). We find that cholesterol transfers easily
between adjacent leaflets belonging to the same bilayer via fast
orientational diffusion (tumbling) in the inter- leaflet disordered
region, while at the same time there is a large free energy cost
against swelling. This fast flip-flop may play an important role in
accommodating the variety of curvatures that would be required in the
three dimensional arrangement of the lipid multilayers in skin, and
for enabling mechanical or hydration induced strains without large
curvature elastic costs.
Actin Polymerization on Supported Cationic Lipid Membranes
George Heath, Benjamin Johnson, Peter D Olmsted, Simon Connell, and Stephen D. Evans,
Biophysical
Journal 105 (2013) 2355-2365.
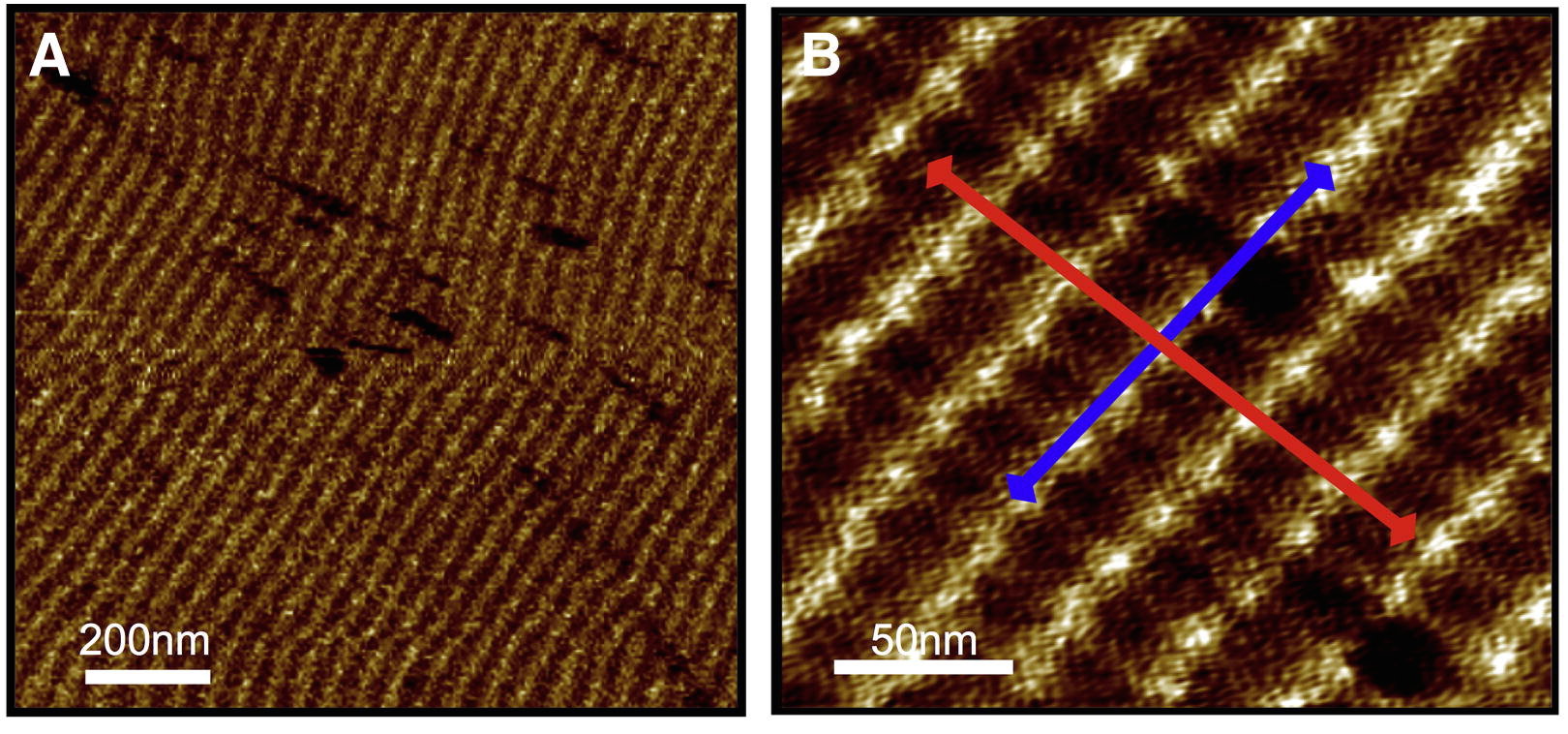 Interactions between the actin cortex and cell membrane are complex
and not completely understood both in terms of actin polymerization
and the effect of actin networks on lipid organization. Using atomic
force microscopy (AFM) and Quartz crystal microbalance with
dissipation (QCM-D) of supported lipid bilayers we investigate the
process of actin polymerization from a non-polymerizing solution via
cationic phospholipids. This polymerization process was studied using
bilayers with varying fractions of charged lipid, different lipid
mobilities and various buffer pH conditions. No interaction was found
between G-actin and neutral lipid bilayers (DOPC or DPPC) both by
QCM-D and AFM. Fluid lipid bilayers containing 18%, or greater,
positively charged lipid polymerize the actin into an almost complete
layer whilst similarly charged bilayers in the gel phase led to the
formation of randomly oriented actin filaments, with lower coverage.
The growth rate on the gel phase bilayers was slow enough to allow AFM
visualisation of the growth of single filaments at conventional scan
rates.
Interactions between the actin cortex and cell membrane are complex
and not completely understood both in terms of actin polymerization
and the effect of actin networks on lipid organization. Using atomic
force microscopy (AFM) and Quartz crystal microbalance with
dissipation (QCM-D) of supported lipid bilayers we investigate the
process of actin polymerization from a non-polymerizing solution via
cationic phospholipids. This polymerization process was studied using
bilayers with varying fractions of charged lipid, different lipid
mobilities and various buffer pH conditions. No interaction was found
between G-actin and neutral lipid bilayers (DOPC or DPPC) both by
QCM-D and AFM. Fluid lipid bilayers containing 18%, or greater,
positively charged lipid polymerize the actin into an almost complete
layer whilst similarly charged bilayers in the gel phase led to the
formation of randomly oriented actin filaments, with lower coverage.
The growth rate on the gel phase bilayers was slow enough to allow AFM
visualisation of the growth of single filaments at conventional scan
rates.
Coexisting gel-fluid phase systems showed localized polymerization starting at the domain edges and growing inwards towards the centre of the ordered domain suggesting the recruitment of positive charges to the more ordered phase to facilitate sustained polymerization. Interestingly, the charge lipid appeared to have sufficient mobility within the gel phase to allow the formation of highly ordered actin layers. Reducing the pH increased the polymerization rate, the number of nucleation events and the total coverage of actin. We propose a mechanism for the bilayer induced polymerization process arising as a result of the localization of oriented G-actin monomers at the membrane surface and thereby increasing the probability of interacting with other G-actin monomers via surface diffusion to create a critical nucleus for filament formation. Filaments appear to be stable for long times (over several hours) and do not depolymerize even when the G-actin is removed from the supernatant - making this a practical approach for creating stable lipid-actin systems via self-assembly.
Lamellar and inverse micellar structures of skin lipids: Effect of templating
Chinmay Das, Peter D. Olmsted, and Massimo Noro,
Physical
Review Letters 111 (2013) 141801
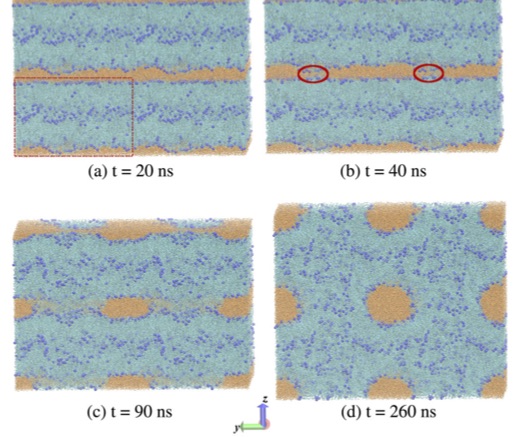 The outermost layer of skin comprises rigid nonviable cells
(corneocytes) in a layered lipid matrix. Using atomistic simulations
we find that the equilibrium phase of the skin lipids is inverse
micellar. A model of the corneocyte is used to demonstrate that
lamellar layering is induced by the patterned corneocyte wall. The
inverse micellar phase is consistent with in vivo observations in
regions where corneocyte walls are well separated (lacunar spaces) and
in the inner layers of skin, and suggests a functional role in the
lipid synthesis pathway in vivo.
The outermost layer of skin comprises rigid nonviable cells
(corneocytes) in a layered lipid matrix. Using atomistic simulations
we find that the equilibrium phase of the skin lipids is inverse
micellar. A model of the corneocyte is used to demonstrate that
lamellar layering is induced by the patterned corneocyte wall. The
inverse micellar phase is consistent with in vivo observations in
regions where corneocyte walls are well separated (lacunar spaces) and
in the inner layers of skin, and suggests a functional role in the
lipid synthesis pathway in vivo.
Critical Point Fluctuations in Supported Lipid Membranes
Simon D Connell, G Heath, Peter D Olmsted,
and Anastasis Kisil,
Faraday Discussions 161 (2013) 91-111.
In this paper, we demonstrate that it is possible to observe many aspects of critical phenomena in supported lipid bilayers using atomic force microscopy (AFM) with the aid of stable and precise temperature control. The regions of criticality were determined by accurately measuring and calculating phase diagrams for the 2 phase Ld-Lo region, and tracking how it moves with temperature, then increasing the sampling density around the estimated critical regions. Compositional fluctuations were observed above the critical temperature (Tc) and characterised using a spatial correlation function. From this analysis, the phase transition was found to be most closely described by the 2D Ising model, showing it is a critical transition. Below Tc roughening of the domain boundaries occurred due to the reduction in line tension close to the critical point. Smaller scale density fluctuations were also detected just below Tc. At Tc, we believe we have observed fluctuations on length scales greater than 10 mm. The region of critically fluctuating 10-100 nm nanodomains has been found to extend a considerable distance above Tc to temperatures within the biological range, and seem to be an ideal candidate for the actual structure of lipid rafts in cell membranes. Although evidence for this idea has recently emerged, this is the first direct evidence for nanoscale domains in the critical region.
Manipulation and sorting of membrane proteins using patterned
diffusion-aided ratchets with AC fields in supported bilayers
MR Cheetham, JP Bramble, DGG McMillan, RJ Bushby, PD Olmsted, LJC Jeuken, and SD Evans,
Soft Matter 8 (2012) 5459-5465.
We present ratchets capable of directing the movement of charged components within supported bilayer lipid membranes. These ratchets make use of asymmetrically patterned features and AC electric fields, and have been demonstrated to transport charged species such as lipids and transmembrane proteins between two reservoirs. Proteins were present in both orientations in the membrane, with only those with their extra-membranous domain orientated away from the glass substrate being mobile. Proteins in the mobile orientation were transported using these ratchets, thereby sorting the two orientations from one another, and creating an area of the membrane containing five times more protein in one orientation than the other.
Concentrating membrane proteins using asymmetric traps and AC
fields
MR Cheetham, JP Bramble, DGG McMillan, L Krzeminski, X Han, BRG Johnson, RJ Bushby, PD Olmsted, LJC Jeuken, SJ Marritt, JN Butt, and SD Evans,
Journal of the American Chemical Society 133
(2011) 6521-6424.
Membrane proteins are key components of the plasma membrane and are responsible for control of chemical ionic gradients, metabolite and nutrient transfer, and signal transduction between the interior of cells and the external environment. Of the genes in the human genome, 30% code for membrane proteins (Krogh et al. J. Mol. Biol. 2001, 305, 567). Furthermore, many FDA-approved drugs target such proteins (Overington et al. Nat. Rev. Drug Discovery 2006, 5, 993). However, the structure-function relationships of these are notably sparse because of di!cul- ties in their puri"cation and handling outside of their membranous environment. Methods that permit the manipulation of membrane components while they are still in the membrane would find widespread application in separation, purification, and eventual structure-function determination of these species (Poo et al. Nature 1977, 265, 602). Here we show that asymmetrically patterned supported lipid bilayers in combination with AC electric fields can lead to efficient manipulation of charged components. We demonstrate the concentration and trapping of such components through the use of a "nested trap" and show that this method is capable of yielding an approximately 30-fold increase in the average protein concentration. Upon removal of the "eld, the material remains trapped for several hours as a result of topographically restricted diffusion. Our results indicate that this method can be used for concentrating and trapping charged membrane components while they are still within their membranous environment. We anticipate that our approach could find widespread application in the manipulation and study of membrane proteins.
Nano-scale mechanical probing of supported lipid bilayers
with atomic force microscopy
K Sheik, C Das, PD Olmsted, and SD Connell
Physical Review E 82 (2010) 041920.
We present theory and experiments for the force-distance curve F(z0) of an atomic force microscope (AFM) tip (radius R) indenting a supported fluid bilayer (thickness 2d). For realistic conditions the force is dominated by the area compressibility modulus kappa_A of the bilayer and, to an excellent approximation, given by F = pi kappa_A R z_0^2 /(2d - z_0)^2. The experimental AFM force curves from coexisting liquid ordered and liquid disordered domains in three-component lipid bilayers are well described by our model, which provides kappa_A in agreement with literature values. The liquid ordered phase has a yieldlike response that we model as due to the breaking of hydrogen bonds.
Undulation instability in a bilayer lipid membrane due to
electric field interaction with lipid dipoles
RJ Bingham, PD Olmsted, and SW Smye
Physical Review E 81 (2010) 051909.
Bilayer lipid membranes (BLMs) are an essential component of all biological systems, forming a functional barrier for cells and organelles from the surrounding environment. The lipid molecules that form membranes contain both permanent and induced dipoles, and an electric field can induce the formation of pores when the transverse field is sufficiently strong (electroporation). Here, a phenomenological free energy is constructed to model the response of a BLM to a transverse static electric field. The model contains a continuum description of the membrane dipoles and a coupling between the headgroup dipoles and the membrane tilt. The membrane is found to become unstable through buckling modes, which are weakly coupled to thickness fluctuations in the membrane. The thickness fluctuations, along with the increase in interfacial area produced by membrane buckling, increase the probability of localized membrane breakdown, which may lead to pore formation. The instability is found to depend strongly on the strength of the coupling between the dipolar headgroups and the membrane tilt as well as the degree of dipolar ordering in the membrane.
Water permeation through stratum corneum lipid
bilayers from atomistic simulations
C Das, PD Olmsted, and MG Noro
Soft Matter 5 (2009) 4549-4555.
Stratum corneum, the outermost layer of skin, consists of keratin filled rigid non-viable corneocyte cells surrounded by multilayers of lipids. The lipid layer is responsible for the barrier properties of the skin. We calculate the excess chemical potential and diffusivity of water as a function of depth in lipid bilayers with compositions representative of the stratum corneum using atomistic molecular dynamics simulations. The maximum in the excess free energy of water inside the lipid bilayers is found to be twice that of water in phospholipid bilayers at the same temperature. Permeability, which decreases exponentially with the free energy barrier, is reduced by several orders of magnitude as compared with phospholipid bilayers. The average time it takes for a water molecule to cross the bilayer is calculated by solving the Smoluchowski equation in the presence of the free energy barrier. For a bilayer composed of a 2:2:1 molar ratio of ceramide NS 24:0, cholesterol and free fatty acid 24:0 at 300 K, we estimate the permeability P = 3.7*10^(-9) cm/s and the average crossing time tau_av= 0.69 ms. The permeability is about 30 times smaller than existing experimental results on mammalian skin sections.
Simulation studies of Stratum Corneum lipid membranes
C Das, PD Olmsted, and MG Noro
Biophysical Journal 97 (2009) 1941-1951.
We present atomistic molecular dynamics results for fully hydrated bilayers composed of ceramide NS-24:0, free fatty acid 24:0 and cholesterol, to address the effect of the different components in the stratum corneum (the outermost layer of skin) lipid matrix on its structural properties. Bilayers containing ceramide molecules show higher in-plane density and hence lower rate of passive transport compared to phospholipid bilayers. At physiological temperatures, for all composition ratios explored, the lipids are in a gel phase with ordered lipid tails. However, the large asymmetry in the lengths of the two tails of the ceramide molecule leads to a fluidlike environment at the bilayer midplane. The lateral pressure profiles show large local variations across the bilayer for pure ceramide or any of the two-component mixtures. Close to the skin composition ratio, the lateral pressure fluctuations are greatly suppressed, the ceramide tails from the two leaflets interdigitate significantly, the depression in local density at the interleaflet region is lowered, and the bilayers have lowered elastic moduli. This indicates that the observed composition ratio in the stratum corneum lipid layer is responsible for both the good barrier properties and the stability of the lipid structure against mechanical stresses.
Manipulation and charge determination of proteins in photopatterned solid supported bilayers
Xiaojun Han, Matthew R Cheetham, Khizar Sheikh, Peter D Olmsted, Richard J Bushby and Stephen D Evans,
Integrative Biology 1 (2009) 205-211.
This work demonstrates the use of deep UV micropatterned chlorotrimethylsilane (TMS) monolayers to support lipid membranes on SiO2 surfaces. After immersing such a patterned surface into a solution containing small unilamellar vesicles of egg PC, supported bilayer lipid membranes were formed on the hydrophilic, photolyzed regions and lipid monolayer over the hydrophobic, non-photolyzed regions. A barrier between the lipid monolayer and bilayer regions served to stop charged lipids migrating between the two. This allows the system to be used to separate charged lipids or proteins by electrophoresis. Either oppositely charged fluorescence labeled lipids [Texas Red DHPE (negative charge) and D291 (positive charge)] or lipids with different charge numbers [Texas Red DHPE (one negative charge) and NBD PS (two negative charges)] can be separated. We have also studied the migration of streptavidin attached to a biotinylated lipid. Negatively charged streptavidin responds to the applied electric field by moving in the direction of electroosmotic flow, i.e. towards the negative electrode. However the direction of streptavidin movement can be controlled by altering the difference in zeta potential between that of the streptavidin (zeta(1)) and the lipid membrane (zeta(2)). If zeta(1) > zeta(2), streptavidin moves to the negative electrode, while if zeta(1) < zeta(2), streptavidin moves to the positive electrode. This balance was manipulated by adding positively charged lipid DOTAP to the membrane. After measuring the average drift velocity of streptavidin as a function of DOTAP concentration, the point where zeta(1) approximate to zeta(2) was found. At this point zeta(1) was calculated to be -9.8 mV which is in good agreement with the value of -13 mV from force measurements and corresponds to a charge of -2e per streptavidin, thus demonstrating the applicability of this method for determining protein charge.
Lipid organization and the morphology of
solid-like domains in phase-separating binary lipid membranes
V Gordon, WCK Poon, S Egelhaaf, PA Beales, ME Cates,
FC MacKintosh, PD Olmsted, and Z, Zhao,
Journal of Physics Condensed Matter 18 (2006) L415-L420.
In multi-component lipid membranes, phase separation can lead to the formation of domains. The morphology of fluid-like domains has been rationalized in terms of membrane elasticity and line tension. We show that the morphology of solid-like domains is governed by different physics, and instead reflects the molecular ordering of the lipids. An understanding of this link opens new possibilities for the rational design of patterned membranes.
Effect of hydrophobic mismatch on phase behaviour of lipid membranes
EJ Wallace, ND Hooper, and PD Olmsted,
Biophysical
Journal 90 (2006) 4104.
We investigate the competing effects of hydrophobic mismatch and chain stretching on the morphology and evolution of domains in lipid membranes via Monte Carlo techniques. We model the membrane as a binary mixture of particles that differ in their preferred lengths, with the shorter particles mimicking unsaturated nonraft lipids and the longer particles mimicking saturated raft lipids. We find that phase separation can be induced upon increasing either the ratio J/kappa of the hydrophobic surface tension J to the compressibility modulus kappa. J/kappa determines the decay length for thickness changes. When this decay length is larger than the system size the membrane remains mixed. Furthermore, increasing the thickness relaxation time can induce transient phase separation.
Phase Coexistence in Mixed Fluid Membranes
JL Harden, FC MacKintosh, and PD Olmsted,
Physical Review E 72 (2005) 011903.
We study the stability and shapes of domains with spontaneous curvature in fluid films and membranes, embedded in a surrounding membrane with zero spontaneous curvature. These domains can result from the inclusion of an impurity in a fluid membrane or from phase separation within the membrane. We show that for small but finite line and surface tensions and for finite spontaneous curvatures, an equilibrium phase of protruding circular domains is obtained at low impurity concentrations. At higher concentrations, we predict a transition from circular domains, or caplets, to stripes. In both cases, we calculate the shapes of these domains within the Monge representation for the membrane shape. With increasing line tension, we show numerically that there is a budding transformation from stable protruding circular domains to spherical buds. We calculate the full phase diagram and demonstrate two triple points of, respectively, bud-flat-caplet and flat-stripe-caplet coexistence.
The kinetics of phase separation in asymmetric membranes
EJ Wallace and PD Olmsted,
Biophysical Journal 88 (2005) 4072-4083.
Phase separation in a model asymmetric membrane is studied using Monte Carlo techniques. The membrane comprises two species of particles, which mimic different lipids in lipid bilayers and separately possess either zero or non-zero spontaneous curvatures. We study the influence of phase separation on membrane shape and the influence of the coupling of composition and height dynamics on phase separation and domain growth, via both the degree of shape asymmetry and relative kinetic coefficients for height relaxation.
Phase behaviour of three component raft mixtures
S Komura, H Shirotori, and PD Olmsted
Journal of Physics Condensed Matter 17 (2005) S2951-S2955.
To describe the lateral phase separation in biological membranes, we propose phenomenological models for binary and ternary lipid mixtures. We consider a coupling between the local lipid composition and internal membrane structure, and its influence on transitions between liquid and gel membrane phases. Assuming that the gel transition temperature of a pure lipid is shifted by the presence of other lipids, we obtained a variety of binary phase diagrams which are in semi-quantitative agreement with the experiments. With the use of the model parameters deduced from the binary cases, we predict the quantitative phase behaviour of the ternary lipid systems.
Lateral phase separation in mixtures of lipids and
cholesterol systems
S Komura, H Shirotori, PD Olmsted, and D Andelman
Europhysics Letters 67 (2004) 321-327.
In an effort to understand ``rafts'' in biological membranes, we propose phenomenological models for saturated and unsaturated lipid mixtures, and lipid-cholesterol mixtures. We consider simple couplings between the local composition and internal membrane structure, and their influence on transitions between liquid and ``gel'' membrane phases. Assuming that the gel transition temperature of the saturated lipid is shifted by the presence of the unsaturated lipid, and that cholesterol acts as an external field on the chain melting transition, a variety of phase diagrams are obtained. The phase diagrams for binary mixtures of saturated/unsaturated lipids and lipid/cholesterol are in semi-quantitative agreement with the experiments. Our results also apply to regions in the ternary phase diagram of lipid/lipid/cholesterol systems.
Instability of Myelin Tubes under Dehydration-M Chen, CF Schmidt, PD Olmsted, and FC MacKintosh Physical Review E64 (2001) 050903.
We report experimental observations of an undulational instability of myelin figures. Motivated by this, we examine theoretically the deformation and possible instability of concentric, cylindrical, multilamellar membrane structures. Under conditions of osmotic stress (swelling or dehydration), we find a stable, deformed state in which the layer deformation is given by dR ~ r^{\sqrt{B_A/(hB)}, where B_A is the area compression modulus, B is the interlayer compression modulus, and h is the repeat distance of layers. Also, above a finite threshold of dehydration (or osmotic stress), we find that the system becomes unstable to undulations, first with a characteristic wavelength of order \sqrt{\xi d_0}, where \xi is the standard smectic penetration depth and d0 is the thickness of dehydrated region.
Instability and front propagation in tweezed lipid bilayer tubules P. D. Olmsted and F. C. MacKintosh, Journal de Physique II 7 (1997) 139-156. (at Journal de Physique or preprint
We study the mechanism of the `pearling' instability seen recently in experiments on lipid tubules under a local applied laser intensity. We argue that the correct boundary conditions are fixed chemical potentials, or surface tensions \Sigma, at the laser spot and the reservoir in contact with the tubule. We support this with a microscopic picture which includes the intensity profile of the laser beam, and show how this leads to a steady-state flow of lipid along the surface and gradients in the local lipid concentration and surface tension (or chemical potential). This leads to a natural explanation for front propagation and makes several predictions based on the tubule length. While most of the qualitative conclusions of previous studies remain the same, the `ramped' control parameter (surface tension) implies several new qualitative results. We also explore some of the consequences of front propagation into a noisy (due to pre-existing thermal fluctuations) unstable medium.
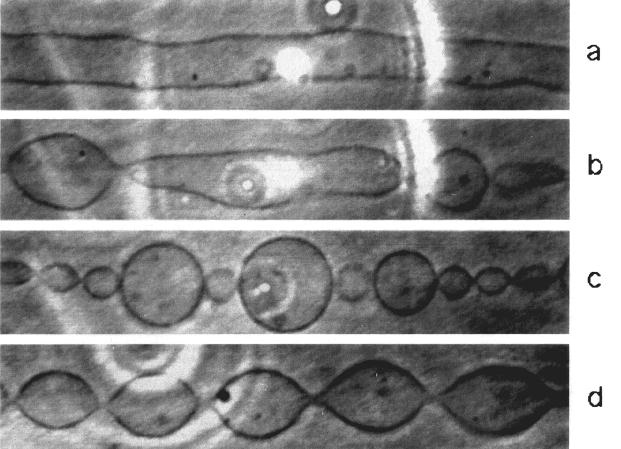 | Successive snapshots of the pearling instability observed by Bar-Ziv and Moses upon submitting a bilayer tubule to laser tweezers. The laser effectively induces a surface tension (dynamically) in the tubule, which then undergoes a variant of the Rayleigh instability. |
Some Properties of Membranes in Nematic Solvents P. D. Olmsted and E. M. Terentjev, Journal de Physique II 6 (1996), 49-56 or cond-mat 951011
The fluctuation spectrum of membranes in nematic solvents is altered by the boundary condition imposed on the bulk nematic director by the curved membrane. We discuss some properties of single and multi-membrane systems in nematic solvents, primarily based on the Berreman-de~Gennes model. We show that: membranes in nematic solvents are more rigid and less rough than in their isotropic counterparts; have a different Helfrich steric stabilization energy, proportional to 1/d^3, and hence a different compression modulus in the lamellar state; and can exhibit phase separation via unbinding during a quench into the nematic state. We also discuss the preparation and possible experimental effects of nematic-mediated surfactant membrane system.
|
Back to the
Olmsted Homestead |
Research Page | peter.olmsted.(at).georgetown.edu |