| Cellular Biophysics |
Axon Motility and Guidance
How do axons sense molecular gradients and use these signals to find their correct
targets? How does the extracellular matrix modulate axon motility? In
collaboration with Herb Geller an the NIH,
we are studying the dynamics of axons navigating in collagen matrices.
We have developed a novel
technology for generating precisely controlled molecular gradients in collagen
gels, and are using it to study the behavior of developing and regenerating axons.
The growing neurons are led by a complex, dynamic structure called a growth cone,
and we are trying to understand how it works as a sensing element. Recently we
have begun investing the biomechanics of the axon outgrowth through live cell
imaging of neurons transfected with fluorescently labeled cytoskeletal proteins.
Funded by NIH. Click on the links for recent
publications
and
movies.
|
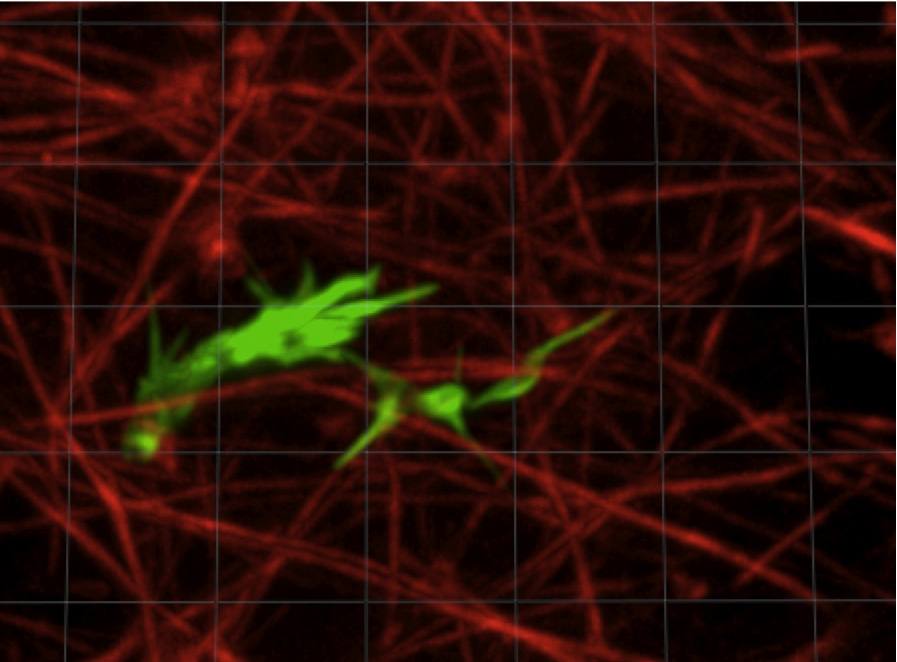 |
|
Axon navigating in a 3D collagen gel. The actin from the axon is
stained in green, the collagen fibrils in red.
|
| Malaria
In collaboration with Professor
Paul Roepe and his group, we are imaging the structure and dynamics of the
malarial parasite after invasion of a
red blood cell. Using a high speed, high resolution confocal microscope, we
have investigated the volume regulation of intracellular compartments in order
to understand the mechanism of drug resistance.
Funding from NIH.
|
 |
| Living P. falciparum parasite inside a human
red blood cell |
Giardia
Giardia lamblia is one of the most prevalent intestinal protozoan pathogens
worldwide, causing widespread infection in areas without access to clean
water. In much of the world, the lack of clean water results in nearly
universal infection of the population by the age of three. After ingestion
of the infective cyst stage by the host, the parasite differentiates
in the lumen of the small intestine into the
trophozoite form, attaches to intestinal epithelial cells (the lower
intestine) and replicates. Its presence often results in severe gastrointestinal
symptoms, including diarrhea, vomiting and weight loss.
In collaboration with Heidi Elmendorf’s lab, we are helping to answer
questions about Giardia’s cytoskeleton and how attaches itself
to the intestinal wall. Ongoing experiments are helping to determine
the mechanism
of attachment employed by Giardia which is believed to be, for the most
part, due either to a suction mechanism or to a gripping mechanism.
Funded by NIH. |
 |
Fluorescence Correlation Microscopy
Fluorescence correlation spectroscopy (FCS) is a technique developed in
the 1970s that is still widely used to study cellular mechanisms and
dynamics. Using a confocal microscope, light intensity fluctuations caused
by fluorescent molecules or microspheres moving through a small measurement
volume (femtoliter) are recorded and analyzed. The autocorrelation of
these fluctuations can be used to determine the diffusion coefficients,
concentrations, mobile fractions, and flow velocities of the fluorescent
particles.
We are developing a massively-parallel version of FCS using a spinning disk
confocal microscope. Whereas a conventional confocal microscope can measure
at only one
diffraction-limited spot at a time, a spinning disk confocal microscope scans
an entire image in one exposure. Each pixel of the image can be used as a location
for FCS, producing ~10^5 independent, spatially-resolved measurements. The
main tradeoff is a limit to the fastest time scales that can be measured, set
by the
fastest camera frame rate--typically around 30 Hz. In a single point confocal
measurement, by contrast, megahertz sampling gives sub-microsecond resolution.
However, the diffusion of large proteins and protein complexes in complex environments
such as the cytoplasm is often slow enough that it can be mapped by this approach.
Funded by NSF.
|
