We have been using reprecipitation to create nanocrystalline wires of various charge transfer co-crystals, in particular phenothiazine:tetracyanoquinodimethane PTZ:TCNQ). Recent theoretical calculations have shown that it could be have a useful organic semiconductor. Organic semiconductors are being heavily investigated for applications in electronics, where they could replace Silicon as the active material in transistors and related devices. While their electronic properties may not match those of Silicon, they have other advantages such as flexibility, low cost, and ease of processing. In addition, many organic semiconductors possess good mobilities for both holes and electrons, making them ambipolar, compared to doped Silicon, which is bipolar. New types of devices have been proposed using ambipolar semiconductors, for example for use radio frequency inverters. In addition, the ambipolar nature may make them useful as charge transport additives in organic photovoltaic devices. SEM images of wires that were freeze-dried following nanoprecipitation show nanowires with diameters ranging from ~100 nm to several microns, and lengths from ten to several hundred microns. We have been characterizing the optical and electronic properties of these materials in collaboration with Prof. Patrick Vora at George Mason University and Prof. Pratibha Dev at Howard University. With support from the Georgetown Environmental Initiative, we are constructing a characterization station with a solar simulator for device efficiency measurements and a wavelength tunable raster system for measuring spatial and wavelength dependence response.

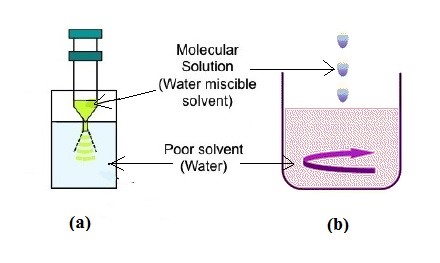
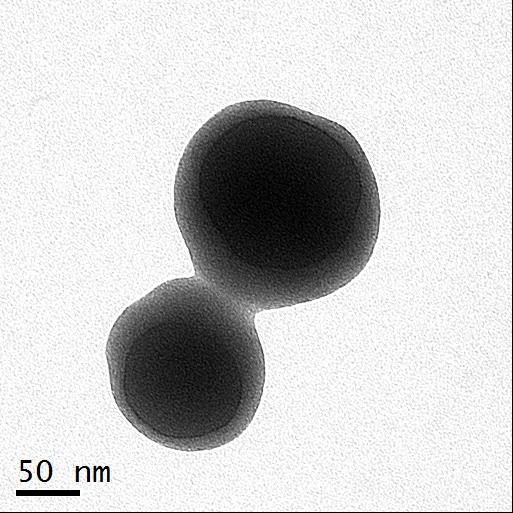 Nanoparticle synthesis
Nanoparticle synthesis
